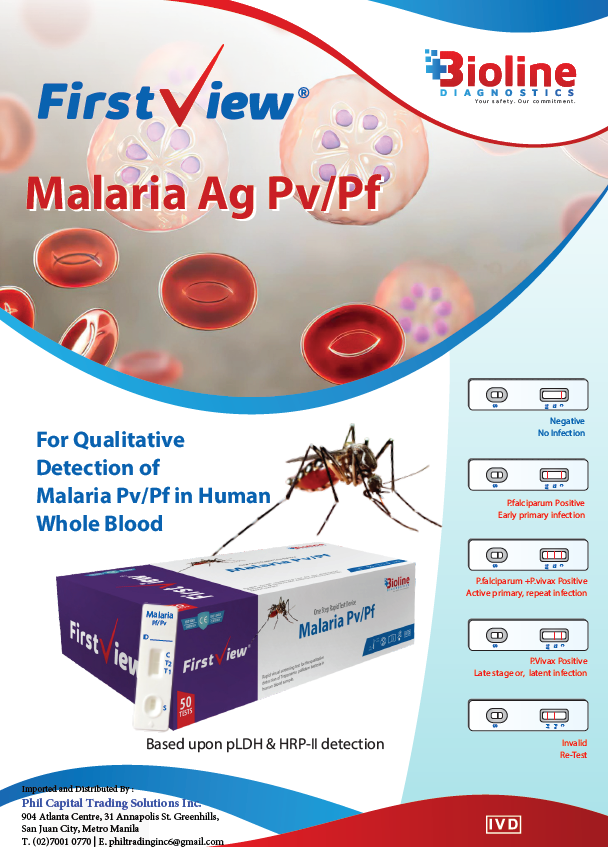
The Malaria Ag Pv/Pf Rapid Test Kit is a qualitative, lateral flow immunoassay designed for the rapid detection and differentiation of Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) antigens in human whole blood. Using dual antigen targets (HRP-II for Pf and pLDH for Pv), this test provides accurate, on-site screening for malaria infection within minutes—without the need for laboratory equipment.
Key Features
- Dual Detection: Detects both P. falciparum and P. vivax antigens simultaneously using HRP-II and pLDH markers.
- Fast Results: Delivers reliable results in just 15–20 minutes.
- High Sensitivity & Specificity: Provides accurate detection for early, active, and latent malaria infections.
- Simple & Portable: Requires no special equipment—ideal for clinical, field, and emergency screening.
- Comprehensive Diagnosis: Differentiates between P. falciparum, P. vivax, or mixed infections.
- Certified Quality: Manufactured under strict ISO 13485 and ISO 9001 standards for consistent performance and reliability.
Interpretation of Results
- Negative: Only control (C) line appears — no malaria infection detected.
- P. falciparum Positive: Control (C) and T1 (Pf) lines appear — early or primary infection.
- P. vivax Positive: Control (C) and T2 (Pv) lines appear — late or latent infection.
- P. falciparum + P. vivax Positive: All three lines (C, T1, T2) appear — mixed or active infection.
- Invalid: No control line — repeat the test with a new device.
Specifications
- Detection Targets: HRP-II (P. falciparum) and pLDH (P. vivax)
- Specimen Type: Whole blood (fingerstick or venipuncture)
- Test Type: Qualitative immunochromatographic assay
- Result Time: 15–20 minutes
- Storage: 2–30°C (room temperature)
- Certifications: ISO 13485, ISO 9001
- Intended Use: In-vitro diagnostic use only
Download Brochure