The Typhoid IgG/IgM Rapid Test Kit is a qualitative, two-step diagnostic assay designed for the detection and differentiation of IgG and IgM antibodies specific to Salmonella typhi in human whole blood, serum, or plasma. This easy-to-use test helps identify both current and past infections, providing clear results in minutes for effective diagnosis and treatment decisions.
Key Features
- Dual Antibody Detection: Simultaneously detects IgG and IgM antibodies to distinguish between early, active, and past infections.
- Fast Results: Provides clear and accurate results within 15–20 minutes.
- Simple Two-Step Procedure: Easy sample application and buffer addition—no laboratory equipment needed.
- Accurate & Reliable: High sensitivity and specificity for rapid on-site diagnosis.
- Versatile Sample Compatibility: Works with whole blood, serum, or plasma samples.
- Certified Quality: Produced under ISO 13485 and ISO 9001 quality management systems for precision and safety.
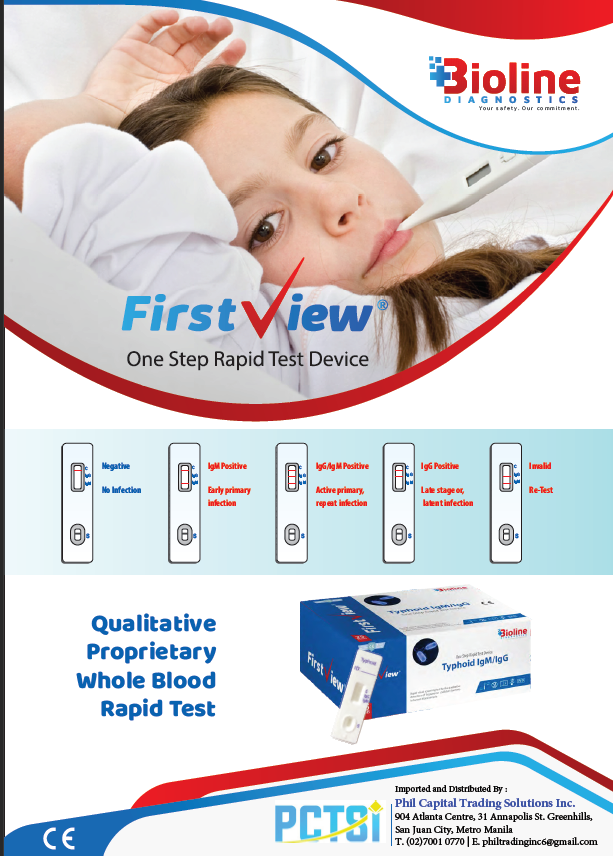
Interpretation of Results
- Negative: Only control (C) line appears — no typhoid antibodies detected.
- IgM Positive: Control (C) and IgM lines appear — indicates early primary infection.
- IgG Positive: Control (C) and IgG lines appear — indicates past or late-stage infection.
- IgG + IgM Positive: All three lines appear — indicates active or repeat infection.
- Invalid: No control line — repeat test with a new device.
Specifications
- Detection Target: IgG and IgM antibodies to Salmonella typhi
- Specimen Type: Whole blood, serum, or plasma
- Test Type: Qualitative immunochromatographic rapid test
- Result Time: 15–20 minutes
- Storage: 2–30°C (room temperature)
- Certifications: ISO 13485, ISO 9001
- Intended Use: In-vitro diagnostic use only
Download Brochure